IPTG (isopropyl-β-D-thiogalactoside) is an analogue of β-galactosidase substrate, which is highly inducible. Under the induction of IPTG, the inducer can form a complex with the repressor protein, So that the conformation of the repressor protein is changed, so that it cannot be combined with the target gene, and the target gene is expressed efficiently. So how should the concentration of IPTG be determined during the experiment? Is the bigger the better?
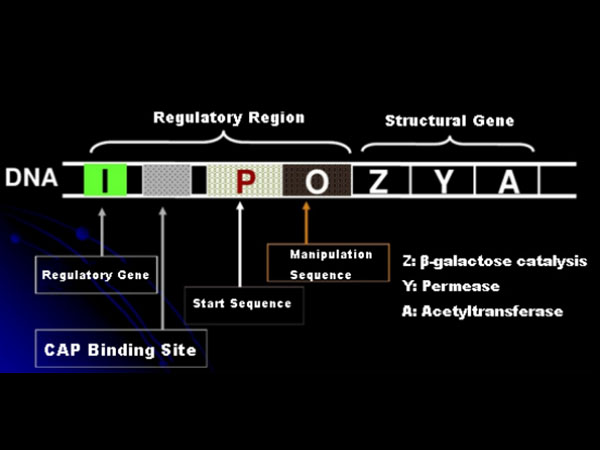
First, let's understand the principle of IPTG induction: E. coli's lactose operon (element) contains three structural genes, Z,Y, and A, which encode β-galactosidase, permease, and acetyltransferase, respectively. lacZ hydrolyzes lactose into glucose and galactose, or into allo-lactose; lacY allows lactose in the environment to pass through the cell membrane and enters the cell; lacA transfers the acetyl group from acetyl-CoA to β-galactoside, which involves removing Toxic effect. In addition, there is an operating sequence O, a starting sequence P and a regulatory gene I. The I gene code is a repressor protein that can bind to the position O of the operator sequence, so that the operon (meta) is repressed and turned off. There is also a binding site for catabolic gene activator protein-CAP binding site upstream of the initiating sequence P. The P sequence, O sequence and CAP binding site together constitute the regulatory region of the lac operon. The coding genes of the three enzymes are regulated by the same regulatory region to achieve the coordinated expression of gene products.
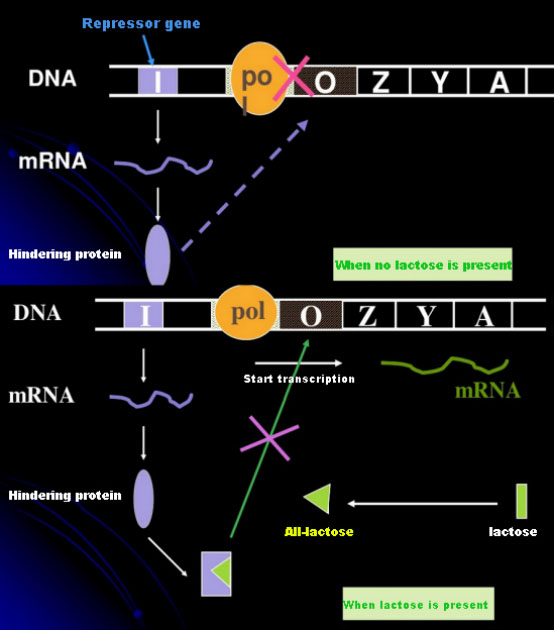
In the absence of lactose, the lac operon (meta) is in a state of repression. At this time, the lac repressor expressed by the I sequence under the control of the PI promoter sequence binds to the O sequence, which prevents RNA polymerase from binding to the P sequence and inhibits transcription initiation; when lactose is present, the lac operon (meta) can be induced In this operon (meta) system, the real inducer is not lactose itself. Lactose enters the cell and is catalyzed by β-galactosidase to be converted to allolactose. The latter, as an inducer molecule, binds to the repressor protein and changes the protein conformation, which leads to the dissociation of the repressor protein from the O sequence and transcription. Isopropylthiogalactoside (IPTG) has the same effect as allolactose. It is a very powerful inducer, which is not metabolized by bacteria and is very stable, so it is widely used in laboratories.
How to determine the optimal concentration of IPTG? Take E. coli as an example.
The E. coli BL21 genetically engineered strain containing the positive recombinant pGEX (CGRP/msCT) was inoculated into LB liquid medium containing 50μg·mL-1 Amp, and cultured overnight at 37°C. The above culture was inoculated into 10 bottles of 50mL fresh LB liquid medium containing 50μg·mL-1 Amp at a ratio of 1:100 for expansion culture, and when the OD600 value was 0.6~0.8, IPTG was added to the final concentration. It is 0.1, 0.2, 0.3, 0.4, 0.5, 0.6, 0.7, 0.8, 0.9, 1.0mmol·L-1. After induction at the same temperature and the same time, 1 mL of the bacterial solution was taken from it, and the bacterial cells were collected by centrifugation and subjected to SDS-PAGE to analyze the influence of different IPTG concentrations on protein expression, and then select the IPTG concentration with the largest protein expression.
After experiments, it will be found that the concentration of IPTG is not as large as possible. This is because IPTG has a certain toxicity to bacteria. Exceeding the concentration will also kill the cell; and generally speaking, we hope that the more soluble protein expressed in the cell, the better, but in many cases when the concentration of IPTG is too high, a large amount of inclusion will be formed. Body, but the amount of soluble protein decreased. Therefore, the most suitable IPTG concentration is often not the bigger the better, but the lower the concentration.
The purpose of induction and cultivation of genetically engineered strains is to increase the yield of the target protein and reduce costs. The expression of the target gene is not only affected by the strain's own factors and the expression plasmid, but also by other external conditions, such as the concentration of the inducer, the induction temperature and the induction time. Therefore, in general, before an unknown protein is expressed and purified, it is best to study the induction time, temperature and IPTG concentration in order to select the appropriate conditions and obtain the best experimental results.
Post time: Dec-31-2021

